과불화옥탄산
package.lua 80번째 줄에서 Lua 오류: module 'Module:Namespace detect/data' not found.
[[분류:짧은 설명을 포함한 스크립트 오류: "pagetype" 모듈이 없습니다.]]
| |

| |
| 이름 | |
|---|---|
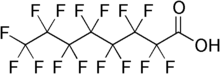
| IUPAC 이름
pentadecafluorooctanoic acid
| |
| 별칭
perfluorooctanoic acid, PFOA, C8, perfluorooctanoate, perfluorocaprylic acid, FC-143, F-n-octanoic acid, PFO
| |
| 식별자 | |
| 스크립트 오류: "list" 모듈이 없습니다. | |
3D 모델 (JSmol)
|
스크립트 오류: "list" 모듈이 없습니다. |
| 3DMet | 스크립트 오류: "list" 모듈이 없습니다. |
| ChEBI | 스크립트 오류: "list" 모듈이 없습니다. |
| ChEMBL | 스크립트 오류: "list" 모듈이 없습니다. |
| ChemSpider | 스크립트 오류: "list" 모듈이 없습니다. |
| DrugBank | 스크립트 오류: "list" 모듈이 없습니다. |
| ECHA InfoCard | 모듈:Wikidata 927번째 줄에서 Lua 오류: attempt to index field 'wikibase' (a nil value). 모듈:Wikidata 927번째 줄에서 Lua 오류: attempt to index field 'wikibase' (a nil value). |
| EC 번호 | 스크립트 오류: "list" 모듈이 없습니다. |
| 식품 첨가물 코드 번호 | 모듈:Wikidata 927번째 줄에서 Lua 오류: attempt to index field 'wikibase' (a nil value). |
| 스크립트 오류: "list" 모듈이 없습니다. | |
| KEGG | 스크립트 오류: "list" 모듈이 없습니다. |
PubChem CID
|
스크립트 오류: "list" 모듈이 없습니다. |
| RTECS 번호 | 스크립트 오류: "list" 모듈이 없습니다. |
| UNII | 스크립트 오류: "list" 모듈이 없습니다. |
CompTox Dashboard (EPA)
|
스크립트 오류: "list" 모듈이 없습니다. |
| 스크립트 오류: "collapsible list" 모듈이 없습니다. | |
| 스크립트 오류: "collapsible list" 모듈이 없습니다. | |
| 성질 | |
| C8HF15O2 | |
| 몰 질량 | 414.07 g/mol |
| 겉보기 | White solid |
| 밀도 | 1.8 g/cm3[1] |
| 녹는점 | 스크립트 오류: "convert" 모듈이 없습니다.[1] |
| 끓는점 | 스크립트 오류: "convert" 모듈이 없습니다.[1] |
| soluble, 9.5 g/L (PFO)[2] | |
| other solvents에서의 용해도 | polar organic solvents |
| 산성도 (pKa) | ~0[3][4][5] |
| 위험 | |
| 주요 위험 | Strong Acid, Causes Burns, Teratogenic, Carcinogenic |
| 물질 안전 보건 자료 | [1] |
| 신호어 | 스크립트 오류: "unstrip" 모듈이 없습니다.스크립트 오류: "Preview warning" 모듈이 없습니다.danger |
| NFPA 704 (파이어 다이아몬드) | <imagemap>
File:NFPA 704.svg|80px|alt=NFPA 704 four-colored diamond poly 300 0 450 150 300 300 150 150 Flammability code 0: Will not burn. E.g. water poly 150 150 300 300 150 450 0 300 Health code 3: Short exposure could cause serious temporary or residual injury. E.g. chlorine gas poly 450 150 600 300 450 450 300 300 Reactivity code 0: Normally stable, even under fire exposure conditions, and is not reactive with water. E.g. liquid nitrogen poly 300 300 450 450 300 600 150 450 Special hazards (white): no code desc none </imagemap> |
| 관련 화합물 | |
관련 compounds
|
Perfluorooctanesulfonic acid (PFOS), Perfluorononanoic acid (PFNA), Perfluorooctanesulfonamide (PFOSA), Trifluoroacetic acid (TFA) |
달리 명시된 경우를 제외하면, 표준상태(25 °C [77 °F], 100 kPa)에서 물질의 정보가 제공됨. | |
| 정보상자 각주 | |
Perfluorooctanoic acid (PFOA) (conjugate base perfluorooctanoate)—also known as C8—is a perfluorinated carboxylic acid produced and used worldwide as an industrial surfactant in chemical processes and as a material feedstock, and is a health concern and subject to regulatory action and voluntary industrial phase-outs. PFOA is considered a surfactant, or fluorosurfactant, due to its chemical structure consisting of a perfluorinated, n-octyl "tail group" and a carboxylate "head group". The head group can be described as hydrophilic while the fluorocarbon tail is both hydrophobic and lipophobic. The tail group is inert and does not interact strongly with polar or non-polar chemical moieties; the head group is reactive and interacts strongly with polar groups, specifically water. The "tail" is hydrophobic due to being non-polar and lipophobic because fluorocarbons are less susceptible to the London dispersion force than hydrocarbons.[6]
PFOA is used for several industrial applications, including carpeting, upholstery, apparel, floor wax, textiles, fire fighting foam and sealants. PFOA serves as a surfactant in the emulsion polymerization of fluoropolymers and as a building block for the synthesis of perfluoroalkyl-substituted compounds, polymers, and polymeric materials. PFOA has been manufactured since the 1940s in industrial quantities.[7] It is also formed by the degradation of precursors such as some fluorotelomers. PFOA is used as a surfactant because it can lower the surface tension of water more than hydrocarbon surfactants while having exceptional stability due to having perfluoroalkyl tail group.[6][8] The stability of PFOA is desired industrially but is a cause of concern environmentally.
A study of workers living near a DuPont Teflon plant found an association between PFOA exposure and two kinds of cancer as well as four other diseases. A positive exposure-response trend for kidney cancer is supported by many studies. PFOA has been detected in the blood of more than 98% of the general US population in the low and sub-parts per billion (ppb) range, and levels are higher in chemical plant employees and surrounding subpopulations. How general populations are exposed to PFOA is not completely understood. PFOA has been detected in industrial waste, stain-resistant carpets, carpet-cleaning liquids, house dust, microwave popcorn bags, water, food, and Teflon (PTFE) products.
As a result of a class-action lawsuit and community settlement with DuPont, three epidemiologists conducted studies on the population surrounding a chemical plant that was exposed to PFOA at levels greater than in the general population. Studies have found correlation between high PFOA exposure and six health outcomes: kidney cancer, testicular cancer, ulcerative colitis, thyroid disease, hypercholesterolemia (high cholesterol), and pregnancy-induced hypertension.[9]
The primary manufacturer of PFOS, the 3M Company (known as Minnesota Mining and Manufacturing Company from 1902 to 2002), began a production phase-out in 2002 in response to concerns expressed by the United States Environmental Protection Agency (EPA).[10] Eight other companies agreed to gradually phase out the manufacturing of the chemical by 2015.[10]
By 2014, EPA had listed PFOA and perfluorooctanesulfonates (salts of perfluorooctanesulfonic acid, PFOS) as emergent contaminants:
PFOA and PFOS are extremely persistent in the environment and resistant to typical environmental degradation processes. [They] are widely distributed across the higher trophic levels and are found in soil, air and groundwater at sites across the United States. The toxicity, mobility and bioaccumulation potential of PFOS and PFOA pose potential adverse effects for the environment and human health.[10]
각주[편집]
- ↑ 1.0 1.1 1.2 1.3 Record of Perfluorooctanoic acid in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 5 November 2008.
- ↑ 인용 오류:
<ref>태그가 잘못되었습니다;Prevedouros2006라는 이름을 가진 주석에 텍스트가 없습니다 - ↑ 스크립트 오류: "Citation/CS1" 모듈이 없습니다.
- ↑ 스크립트 오류: "Citation/CS1" 모듈이 없습니다.
- ↑ 스크립트 오류: "Citation/CS1" 모듈이 없습니다.
- ↑ 6.0 6.1 스크립트 오류: "Citation/CS1" 모듈이 없습니다.
- ↑ 스크립트 오류: "Citation/CS1" 모듈이 없습니다.
- ↑ 인용 오류:
<ref>태그가 잘못되었습니다;Salager2002라는 이름을 가진 주석에 텍스트가 없습니다 - ↑ 스크립트 오류: "Citation/CS1" 모듈이 없습니다.
- ↑ 10.0 10.1 10.2 스크립트 오류: "citation/CS1" 모듈이 없습니다. Fact sheet.
This article "과불화옥탄산" is from Wikipedia. The list of its authors can be seen in its historical and/or the page Edithistory:과불화옥탄산. Articles copied from Draft Namespace on Wikipedia could be seen on the Draft Namespace of Wikipedia and not main one.
